Glass ionomer cement is the generic name for a group of materials produced by the reaction of silicate glass powder and polyalkenoic acid. Initially, this dental cement was intended for use in aesthetic restorations for anterior teeth, especially for class III and V cavities.
However, given its adhesion to the tooth structure and its effectiveness in preventing caries, it has been extended to other uses such as: cementing agent, fissure sealant, coating, stump reconstruction and immediate restorations, among others.
Want to find out everything there is to know about this type of dental cement? You've come to the right place!
What is glass ionomer cement made of?
Over time, the components of dental cements have undergone many changes, and today, conventional cement is composed of:
- Powder: silica, alumina, fluorides
- Liquid: polyacrylic acid, itaconic acid, tartaric acid.
When both components are mixed (the mixture must be quick, between 20 and 30 seconds at a powder/liquid ratio of 1.5:1) into a paste, the acid etches the surfaces of the glass particles, releasing calcium, sodium, aluminium and fluoride ions into the aqueous medium.

Glass ionomers have an acid-base chemical reaction, in which there is an ion exchange between Ca and the carboxyl group. This setting reaction begins when the liquid comes into contact with the powder. H+ ions attack the glass particles, releasing Ca, Al and F, breaking down the glass through the action of the acid and forming a silica gel.
Next, the Ca reacts with the polyacids and then with the Al. This polyacrylate metal salt begins to precipitate and gelation begins until hardening. Water is an important component in setting as it acts as a plasticiser, reducing rigidity. Therefore, during placement on the tooth, it should not be allowed to dry out as this can contribute to brittleness and excess water promotes dissolution.
Among its advantages are the release of fluoride, which promotes bacteriostatic activity, greater compressive strength than zinc phosphate, similar tensile strength, easy handling and translucency. The high molecular weight of its acid component initially has an acidic pH but quickly increases after mixing begins, preventing pulp toxicity. However, it is highly soluble in moisture, therefore requiring complete isolation.
Classification of glass ionomer cements:
- Glass ionomer cement.
- Resin-modified glass ionomer cements: the moisture sensitivity and low initial strength of GIC are the result of acid-base reaction.
- Polyacid-modified composite resins (compomers).
- Fluoride-releasing composite resins.
Classification of glass ionomer cements according to their use:
- Type I: cemented for fixed restoration.
- Type II: aesthetic or reinforced restoration: they lack strength and therefore cannot withstand high concentrations of loads that promote fractures.
- Type III: cavity protectors.
Take a look at the best-selling glass ionomer cements:
See all glass ionomer cements!
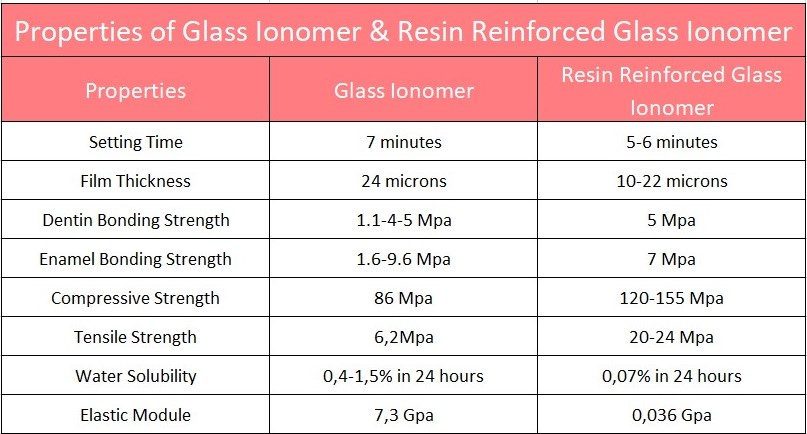
Resin-reinforced glass ionomer cements:
These cements were created with the aim of offering better aesthetics and chemical stability. To improve their adhesion capacity, the former was used to remove dentinal debris, improving the penetration of the ionomer, which has a viscous consistency and, as a result, forms a better hybrid layer.
It has two types of hardening: the typical reaction of acid-base ionomer and that of photo-activated resins. Its components include:
- Powder: Silica, aluminium, fluoride, photoinitiators
- Liquid: Polyacrylic acid, carboxyl copolymers, hydroxyethyl methacrylate (HEMA), water.
If you liked this post, we recommend that you keep an eye out for the following article on dental cements, and don't forget to follow us on social media to stay up to date on offers, news, and promotions in the dental sector. See you soon!